Pseudo-Lignin Formation during Dilute acid Pretreatment for Cellulosic Ethanol-Juniper Publishers
Juniper Publishers
Abstract
Dilute acid-based pretreatment represents one of
the most important pretreatment technologies to reduce biomass
recalcitrance and it has been successfully applied to a wide range of
feedstocks. During this type of pretreatment, the relative lignin
content usually increases partially due to the loss of carbohydrates.
More importantly, it has been reported that the increase of lignin
content after dilute acid pretreatment is mainly due to the formation of
pseudo-lignin. The exact reaction mechanisms leading to the formation
of pseudo-lignin is still under investigation. However, it has been
proposed that rearrangement of hydroxymethylfurfural (HMF) or furfural
can produce aromatic type of compounds which can further undergo
polymerization reactions to from a lignin-like polyphenolic structures
termed as pseudo-lignin. This mini-review mainly covers recent advances
in understanding the fundamentals of pseudo-lignin formation during
dilute acid pretreatment, the impact of its formation on enzymatic
hydrolysis, and how to suppress its formation during dilute acid
pretreatment.
Keywords: Biomass recalcitrance; Pseudo-lignin; Dilute acid pretreatment; Enzymatic hydrolysis; Hydroxymethylfurfural; furfuralAbbreviations: MW: Molecular Weight; Mw: Weight-average molecular weight; Mn: Number-average molecular weight; BTO: 1,2,4-benzenetriol; DMSO: Dimethyl Sulfoxide; HMF: Hydroxymethylfurfural
Introduction
Pharmaceutical excipients
Increasing global energy demand and environment
concerns have led to rapid development of converting renewable resources
such as lignocelluloses biomass to biofuels such as cellulosic ethanol
[1]. Relatively high costs associated with the bioprocess and the
sub-optimal yield of ethanol production remains a great challenge due to
the natural resistance of the plant cell wall to enzymatic
deconstruction. As a result, a chemical or physical pretreatment is
usually required prior to the enzymatic hydrolysis step to disrupt the
lignin-hemicellulose matrix and increase cellulose accessibility, which
subsequently increases the following hydrolysis efficiency [2].
Over the past decades, different pretreatment
technologies have been developed to reduce biomass recalcitrance [3].
Among all the available pretreatment technologies up-to-date, dilute
acid-based pretreatment using a variety of acids including sulfuric
acid, nitric acid, or hydrochloric acid remains as one of the most
important technologies for cellulosic ethanol. It is normally performed
with acid concentration less than 4wt% over a wide range of temperature
(120 to 210 °C) [4]. It also has been applied on a wide range of
feedstock such as poplar [5], switch grass [6], wheat straw [7], rice
straw [8], bagasse [9], maize stems [10], and corn stover [11]. It is
well known that this acidic type of pretreatment, in the absence of an
organic solvent, is less effective in terms of lignin removal, and in
fact, the relative content of Klason lignin is normally found to be
increased after dilute acid pretreatment. For example, Foston et al.
reported that the Klason lignin content significantly increased from
~25% to ~40% after dilute acid pretreatment of Populus [6]. This is of
course partially due to the loss of carbohydrates especially the
hemicellulose during pretreatment. However, Sannigrahi et al. reported
that the formation of pseudo-lignin by the dehydration and
polymerization of carbohydrates should be responsible for this unusual
increase of lignin content [12]. In addition, it was also reported by Li
et al. that only ~50% of the Klason lignin extracted from a hot water
pretreated aspen was actual lignin [13].
Understanding the fundamentals of pseudo-lignin chemistry
is important from the bioconversion process perspective.
Optimization of current pretreatment technologies is often
guided b carbohydrate loss during pretreatment and lignin
residue content and/or structure after pretreatment. Lignin is
well known to effect enzymatic hydrolysis negatively due to its
physical barrier role and its unproductively binding to enzymes
[14,15]. Therefore, development of novel low pH pretreatments
with diminished pseudo-lignin formation will significantly
reduce the enzyme loadings required for an efficient enzymatic
hydrolysis, hence improve the overall process economics. In
conclusion, the formation of pseudo-lignin during any low pH
pretreatment is unfavorable as it originates from carbohydrate
degradation and more importantly, it may be even more
detrimental to enzymatic hydrolysis compared to native lignin.
This mini-review highlights recent advances in understanding
the fundamentals of pseudo-lignin formation, the impact of its
formation on enzymatic hydrolysis rate and yield, and how to
suppress its formation during pretreatment.
Structural Characterization of Pseudo-Lignin
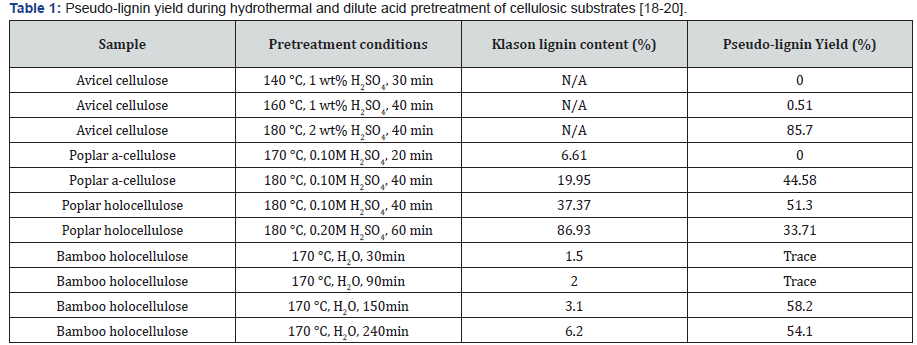
For characterization purposes, pseudo-lignin is normally
produced and isolated from dilute acid pretreated lignin-free
materials such as a-cellulose and holocellulose. Table 1 shows
some typical yields of pseudo-lignin isolated from different
resources under different pretreatment conditions. A variety of
analytical techniques including GPC, FTIR, NMR, SEM have been
utilized to characterize pseudo-lignin. Molecular weight (MW) of
isolated pseudo-lignin from different resources is shown in Table
2. In general, the MW of pseudo-lignin is much lower than that of
milled wood lignin. For example, the weight average MW (Mw)
of milled poplar lignin and pseudo-lignin derived from dilute
acid pretreated poplar holocellulose at 180 °C were found to be
10002g/mol and 5050g/mol, respectively [16,17]. Pretreatment
severity was not found to be a huge impact factor on the MW
of pseudo-lignin (Table 2) [18-20]. In addition, MW of pseudolignin
derived from dilute acid pretreated holocellulose was
found larger than that of pseudo-lignin extracted from pretreated
a-cellulose (Table 2) [16]. FTIR and 13C NMR analysis were also
used to provide additional information on the chemical structure
of pseudo-lignin, which indicated that pseudo-lignin was mainly
composed of hydroxyl, carbonyl and aromatic structures
[12]. These results clearly indicated that pseudo-lignin was
a polyphenolic, lignin-like material with aliphatic, aromatic,
and carbonyl structures derived from cellulose/hemicellulose
fragments released during acid hydrolysis reactions.
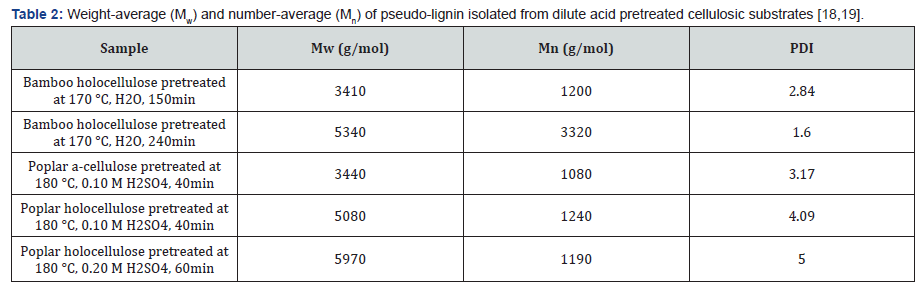
Lignin can be redistributed during dilute acid pretreatment,
leading to the formation of lignin droplets of various
morphologies [10,21-24]. During the dilute acid pretreatment,
lignin was reported to coalesce on plant cell wall and then
migrate into the bulk liquid phase in form of droplets or balls
[21]. This kind of lignin aggregation normally requires the
pretreatment temperature to exceed the lignin phase transition
temperature. Similar to this kind of re-deposit lignin droplets,
pseudo-lignin can be also exist as discrete spherical droplets on
the surface of pretreated holocellulose with a range of sizes from
0.3 to 8.0mmm [12]. Figure 1 illustrates a SEM image of pseudolignin
deposition on surface of poplar holocellulose during dilute
acid pretreatment.
Reaction Mechanisms Leading to the Formation of Pseudo-Lignin
The exact mechanisms leading to the formation of
pseudolignin
are still under investigation due to the complexity of
pseudo-lignin structure and the heterogeneity of reaction
sources and media. However, the presence of high proportions
of unsaturated carbons in pseudo-lignin structure strongly
indicated that acid hydrolysis of carbohydrates polymers to
their corresponding monosaccharide’s and the subsequent
dehydration and fragmentation of sugars probably took place
during the acid pretreatment. Hydroxymethylfurfural (HMF)
and furfural can be produced from 6 and 5-carbon sugars
such as glucose and xylems via acid catalyzed dehydration
reactions [25,26]. HMF and furfural can be further subjected to
rearrangements to produce other aromatic compounds which
might be the key intermediates for pseudo-lignin formation. For
example, 3,8-dihydroxyl-2-methylchromone was reported as
one of the main aromatic products in the acidic degradation of
xylems [27]. Similarly, hydrolytic ring-opening reaction of HMF
was reported to generate 1,2,4-benzenetriol (BTO) in yields of
46% [28]. These intermediates can be then converted to pseudolignin
via polymerization/polycondensation reactions. For
instance, it has been reported that BTO could react with HMF
or furfural to produce a three-dimensional polymer via acid
catalyzed aromatic electrophonic substitution [18]. Figure 2
summarized the reaction mechanisms mentioned above, which
highly suggested presence of acid and high temperatures are
probably two crucial conditions for the pseudo-lignin formation.
Understanding the reaction pathways leading to the formation of
pseudo-lignin will provide insights into how to suppress pseudolignin
generation, though much work still needed to be done.

Impact of Pseudo-Lignin on Enzymatic Hydrolysis
Li and co-workers reported that the lignin droplets
deposited on the surface of plant cell wall significantly inhibited
cellulose hydrolysis, mainly through surface blockage [21].
Their study indicated the nonspecific binding of lignin droplets
to enzymes was not the key source of inhibition. Similar to the
lignin redeposit droplets, pseudo-lignin formed during dilute
acid pretreatment can also exist as discrete spherical droplets
on the surface of pretreated materials, and its formation is
obviously not desired due to the fact that pseudo-lignin can
directly decrease cellulose accessibility by blocking the surface
binding sites. On the other hand, pseudo-lignin is also known
to unproductively bind to cellulase and inhibit its action [29].
Kumar et al. studied the enzymatic hydrolysis of Avicel cellulose
mixed with pseudo-lignin derived from pure xylose, and it was
reported that even a small amount of pseudo-lignin addition
could have a noticeable negative impact on enzymatic hydrolysis
yield [20]. Further protein adsorption experiments revealed
that pseudo-lignin bound to enzymes unproductively. A recent
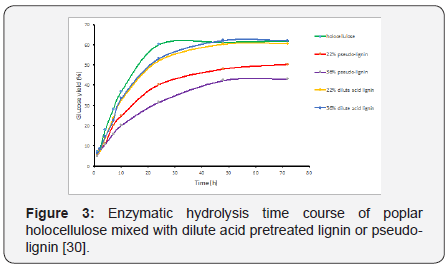
study by Hu et al. further demonstrated the formation of pseudolignin
needed to be avoided as they proved that pseudo-lignin
is much more detrimental to enzymatic hydrolysis than regular
dilute acid pretreated lignin (Figure 3)[30]. More specifically,
dilute acid pretreated lignin only inhibited enzymatic hydrolysis
in its initial stage and had a nearly negligible impact on the
overall conversion percentage after 48 h as shown in Figure
3, whereas pseudo-lignin addition could decrease enzymatic hydrolysis yield up to 25% [30]. It is worth mentioning pseudolignin
is insoluble in water, therefore the hydrophobic structural
functionality of pseudo-lignin is probably responsible for its
nonproductive association with enzymes.
Suppression of Pseudo-Lignin Formation during Dilute Acid Pretreatment
Lignin redeposits droplets and pseudo-lignin only can
be formed at elevated temperatures, therefore reducing
the pretreatment severity is obviously one of the ways to
reduce or avoid the formation of pseudo-lignin. However,
as the pretreatment severity decreases, so do the efficient
of pretreatment in terms of hemicellulose removal and
cellulose accessibility increase. Compared to the typical batch
pretreatment, flow through reactor system was shown to
dramatically increase lignin removal as much as over 90% [31].
More importantly, its ability to constantly remove lignin into the
aqueous phase effectively restricts the condensation reactions.
As a result, flow through pretreatment can reduce the chances
of pseudo-lignin formation [31,32].Oxidative polymerization
seems to play an important role in pseudo-lignin formation
based on the proposed pathway, therefore performing dilute
acid pretreatment under non-oxygen environment could be
another possible alternative method. It was also reported that
introduction of dimethyl sulfoxide (DMSO) to the acidic medium
could effectively suppress HMF productions which is one of key
intermediates during the pseudo-lignin formation [33]. A recent
study modified a series of dilute acid pretreatment by using N2,
surfactant Tween-80, or DMSO-water mixture as the reaction
medium to test these hypotheses for new methods of suppressing
pseudo-lignin formation without significantly reducing the
pretreatment severity [34]. As shown in Table 3, addition of N2
was not effective in terms of pseudo-lignin suppression although
extra oxygen significantly facilitated pseudo-lignin formation
as expected. Apparently, introduction of DMSO significantly
reduced the pseudo-lignin content by ~30%. The coordination of
HMF with water can be reduced in the presence of DMSO due to
the stronger interaction of DMSO oxygen with water [35]. From
the reaction mechanism perspective, the reduction of HMFwater
coordination could protect the HMF molecule from further
reactions to form pseudo-lignin [34].
Conclusion
Lignin-like materials originated from acid catalyzed
dehydration of carbohydrate, termed pseudo-lignin, are
responsible for the increased Klason lignin content after acidbased
biomass pretreatment. Pseudo-lignin can be deposit on
the surface of biomass during the dilute acid pretreatment in
forms of discrete spherical droplets or balls, and it is detrimental
to the subsequent enzymatic hydrolysis, even more detrimental
than dilute acid pretreated lignin. Therefore, it became essential
to develop techniques to effectively impeded pseudo-lignin
formation. Current ongoing pseudo-lignin researches are still
quite limited to carbohydrate-derived pseudo-lignins, it is quite
possible that lignin would somehow react with carbohydrate
degradation products vis polycondensation reactions,
contributing to the yield of pseudo-lignin. The understanding
of the most fundamental chemistry associated with pseudolignin
formation is crucial for the future bioethanol production.
Therefore, future work is much needed to fully unlock the secret
of pseudo-lignin chemistry.
Acknowledgement
This manuscript has been authored by UT-Battle, LLC under
contract no. DE-AC05-00OR22725 with the U.S. Department of
Energy. The publisher, by accepting the article for publication,
acknowledges that the United States Government retains a nonexclusive,
paid-up, irrevocable, worldwide license to publish
or reproduce the published form of this manuscript, or allow
others to do so, for United States Government purposes. The
Department of Energy will provide public access to these results
of federally sponsored research in according with the DOE Public
Access Plan (http://energy.gov/downloads/doe-public-accessplan).
This study was supported and performed as part of the
BioEnergy Science Center (BESC). The BESC is a U.S. Department
of Energy BioEnergy Research Center supported by the Office
of Biological and Environmental Research in the DOE Office of
Science.
To
read more articles in JOJ Sciences
Please
Click on: https://juniperpublishers.com/jojs/index.php



Comments
Post a Comment